Jeff Ortega, Ph.D., Director of Research, ZPower, LLC
Ross Dueber, Ph.D., President and CEO, ZPower, LLC
Silver-zinc batteries have the highest theoretical specific energy (Wh/kg) and energy density (Wh/L) of all rechargeable battery technologies available commercially today. Rechargeable silver-zinc batteries have been successfully used for decades in military and aerospace applications where high energy and power density are required. The electrochemical reaction involves the oxidation of zinc to zinc oxide and the accompanying reduction of silver(II) oxide to metallic silver. The reaction at the AgO cathode involves a two-step oxidation (1.8 V and 1.5 V) of two water molecules to form hydroxide anions that migrate to the anode where they oxidize the metallic Zn to form the soluble zincate species before precipitation of the zinc oxide.
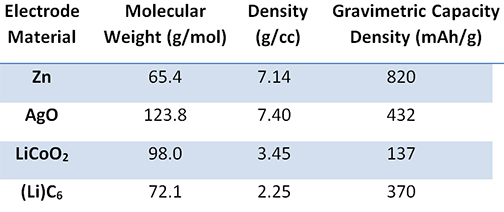
The electrolyte is an aqueous system containing 35 to 45 percent by weight potassium hydroxide, and the separator has historically been cellophane. Because rechargeable silver-zinc batteries use an aqueous electrolyte, they may pose a reduced flammability hazard when compared with some Li-ion batteries. Therefore, silver-zinc batteries are safer for both the user and the environment. The nominal operating voltage is 1.65 V with end-of-discharge and end-of-charge being 1.2 V and 2.0 V, respectively. Figure 1 shows a comparison of the published literature values for the silver-zinc battery energy density and specific energy with respect to other commercial secondary battery chemistries. In the figure, it can be seen that silver-zinc batteries have the highest specific energy and energy density ranges when compared to the other rechargeable chemistry solutions available on the market, including Li-ion. This increased performance stems from the inherently higher material and capacity densities of the electrodes (shown in Table 1).
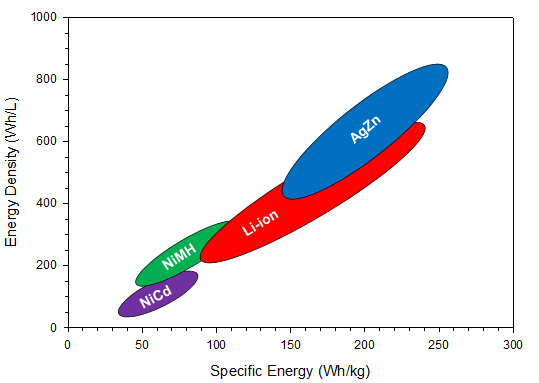
Silver-zinc historically hasn’t achieved widespread commercial use due primarily to short cycle life and high cost. New rechargeable silver-zinc batteries developed by ZPower, however, have addressed the limited cycle life issue through 15 years of R&D and now offer a solution today with cycle life comparable to lithium-ion. The high cost of silver remains an issue and is one of the reasons for focusing upon small button and coin batteries where precious metal cost isn’t exorbitant.
Global battery technology improvement efforts currently focus mainly upon large format batteries for transportation and energy storage with power density, longevity, and cost being the emphasis. At the opposite end of the size spectrum, relatively little attention has been placed upon miniature batteries for electronics and medical applications. The miniature battery segment is where silver-zinc technology offers distinct advantages over every other rechargeable battery technology, even lithium-ion.
Miniature Battery Comparison
The cell performance data used in this report for comparing different commercially available battery chemistries were found on the companies’ websites. The reported energy densities from the different battery manufacturers are compared with respect to size in Figure 2 and with respect to discharge energies in Figure 3.
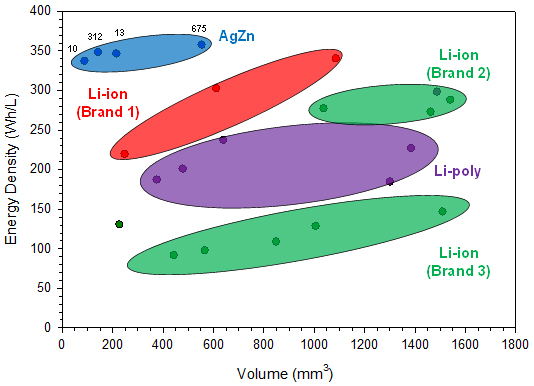
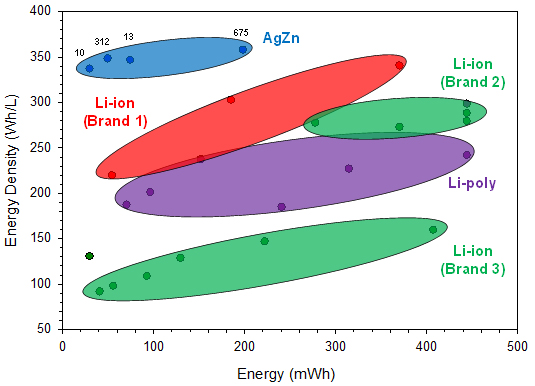
Figures 2 and 3 focus on the smaller sizes that are more comparable to the silver-zinc button cell sizes currently available, up to 600 mm3 and 200 mWh, respectively.
From the performance data plotted in the above two figures, it can be seen that the four silver-zinc button cell batteries deliver greater energy density than the equivalently sized rechargeable Li-ion batteries available on the market today. In fact, there are no rechargeable Li-ion options at volumes less than 200 mm3, whereas there are two silver-zinc solutions. For the other two silver-zinc button cell sizes, due to the higher intrinsic material and capacity density of the electrode materials, they clearly exhibit greater energy density performance than the similarly sized rechargeable Li-ion batteries.
Also, with regard to cycling data for the Li-ion cells published in their specification sheets, they are given with a condition of only being able to maintain above 80 percent of that capacity for a minimum of 200 cycles. Typically, Li-ion cells only deliver the rated capacity for the first few cycles, and then rapidly fall to between 85 to 90 percent within the first 100 cycles. Furthermore, the capacity of the Li-ion cells generally plateaus between 80 to 85 percent of their advertised rated capacity before the 200th cycle. In comparison, the silver-zinc button cells maintain greater than 98 percent of their advertised rated capacity for over 300 cycles. It is again clear that AgZn offers a significantly greater capacity density and cycle life performance over current rechargeable Li-ion solutions.
Since the Li-ion battery involves the intercalation of Li-ions in and out of their electrode material, they are heavily dependent on interfacial surface area between electrodes. Therefore, the performance of a Li-ion button cell is directly dependent on the number of layers from how the electrodes are wound together within a jelly-roll configuration; or for the case of the case of the Li-poly cells, the number of electrode layers that can be stacked. In the case of Li-ion coin cells where the jelly-roll configuration is not an option, a larger diameter with shorter height form factor is preferred. These are the reasons for the spread of Li-ion performance shown in Figures 1 and 2. However for silver-zinc, because of the greater intrinsic electrode materials densities, AgZn battery chemistry is not as sensitive to interfacial surface area, and they can maintain a much higher energy density for these miniature battery sizes (<600 mm3) with their standard planar arrangement, resulting in a distinct advantage in energy density for the silver-zinc batteries at these smaller sizes. Therefore, silver-zinc electrodes can be utilized in a variety of form factors without a loss in performance.
For more information, contact ZPower, LLC at http://zpowerbattery.com.







